trending topics
market reports
-

Registration Now Open: MEDICAL JAPAN 2026 OSAKA – Western Japan’s Largest Healthcare Trade Show
2026-02-10
-

MEDICAL JAPAN 2025 OSAKA Returns to Showcase Global Innovations
2025-02-17
-

Visit MEDICAL JAPAN 2023 TOKYO and take full advantage of the business opportunities!
2023-09-01
-
US to distribute 400 million free N95 masks at CVS, Walgreens in COVID fight
2022-01-21
-

Ethiopia receives additional 2.2 mln doses of Chinese-donated COVID-19 vaccines
2022-01-21
-
Hong Kong researchers say they develop novel material able to kill COVID-19 virus
2022-01-14
-

10 million more Chinese doses on way for Kenya
2022-01-14
-

Sino-African ties on track for a brighter future
2022-01-07
-
Efforts urged to boost COVID-19 vaccine production capacity in poor countries
2022-01-07
-
UAE approves Sinopharm's new protein-based COVID-19 vaccine
2022-01-07
China announces conditional market approval for first COVID-19 vaccine
2020-12-31
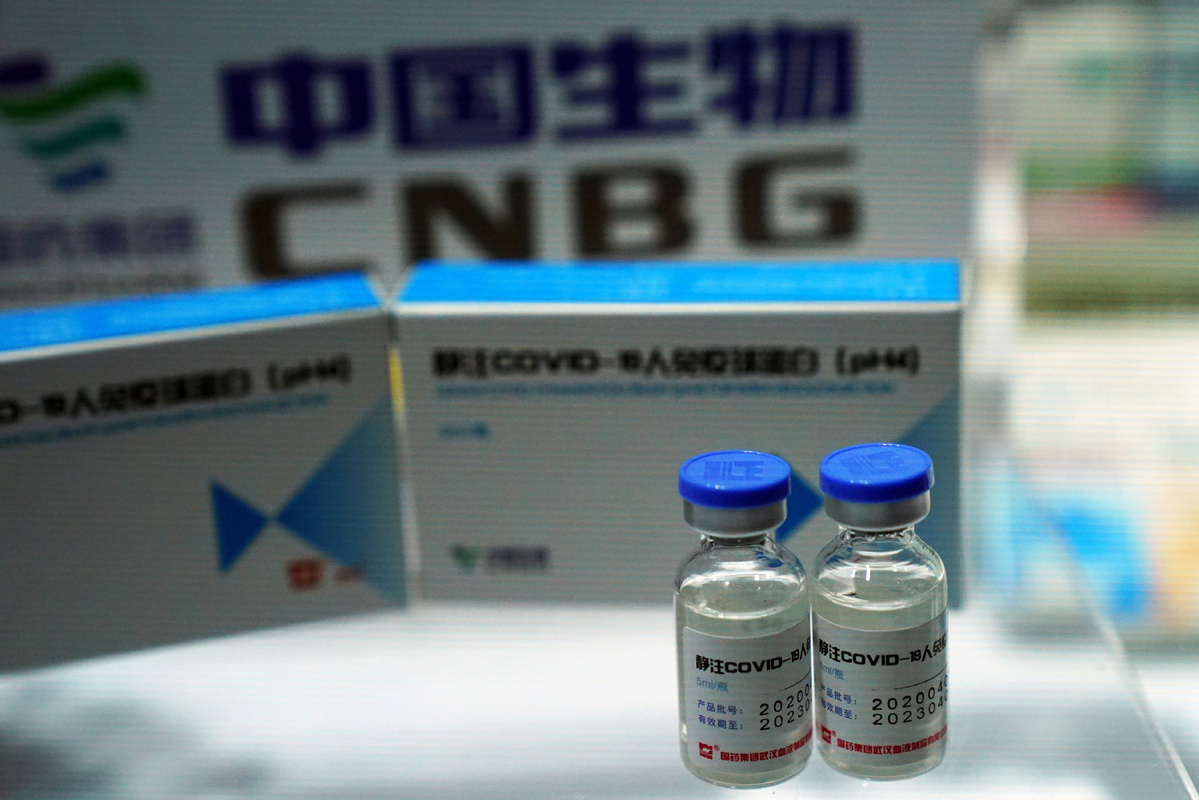
A coronavirus disease (COVID-19) Human Immunoglobulin for Intravenous Injection from China National Biotec Group (CNBG). [Photo/Agencies]
China announced on Thursday it has granted conditional market approval for its first COVID-19 vaccine.
The vaccine was approved by China's top drug regulator on Wednesday night.
China announced on Thursday it has granted conditional market approval for its first COVID-19 vaccine.
The vaccine was approved by China's top drug regulator on Wednesday night.
(China Daily)
label :



 My Member
My Member Message Center
Message Center